
I have always found this topic in physics strange and even magical. It seems so detached from the real world that we know,. Yet, it can explain so many things about atoms, electrons and photons, and be used to calculate and predict many things about them accurately.
So what is strange and magical about quantum physics? First, it says that the tiny particles of electrons are actually waves. Then it says that light wave is actually particles. And all particles are actually waves. All waves are actually particles.
??
We may think of a particle like a small stone, and a wave like water wave. A water wave certainly does not look like a lump of particle, and no way a stone can look like a wave.
So what made scientists conclude that light is actually made up of lumps of particles, after we have seen very convincing interference patterns showing that light is definitely a wave that can spread out?
Two of the earliest and most important reasons: photoelectric effect, and the line spectrum.
Photoelectric Effect
Lets start with the photoelectric effect. This is the effect that when you shine light of certain wavelengths on some metals, electrons can jump out of the metals.
This was an exciting new discovery around the start of the 20th century. Scientists started doing experiments to try and understand more about it. The basic experimental setup is a glass vacuum tube, with two metal conductors on two ends
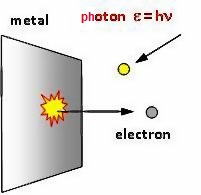
For many years before that, scientists had known that if they apply a very hght voltage, like thousands of volts, an electric current can jump through the vacuum. If they just apply a few volts to the conductors, no current can flow.
This time, they found something very surprising. They found that for certain metals, if you shine light of certain wavelengths on the negative conductor, a current can flow even if you just apply a few volts !
A few volts instead of a few thousand volts! A new toy, a new discovery, and maybe a few Nobel prizes! A Nobel prize was eventually award to Albert Einstein in 1921 for his explanation of this "photoelectric effect".
Thanks to Einstein, we now have to learn the basics of this in physics.
After trying many different metals for the electrodes, with many different voltages across them, many different frequencies of light, many different intensities, the scientists started to see some patterns. The experiment looks something like this.
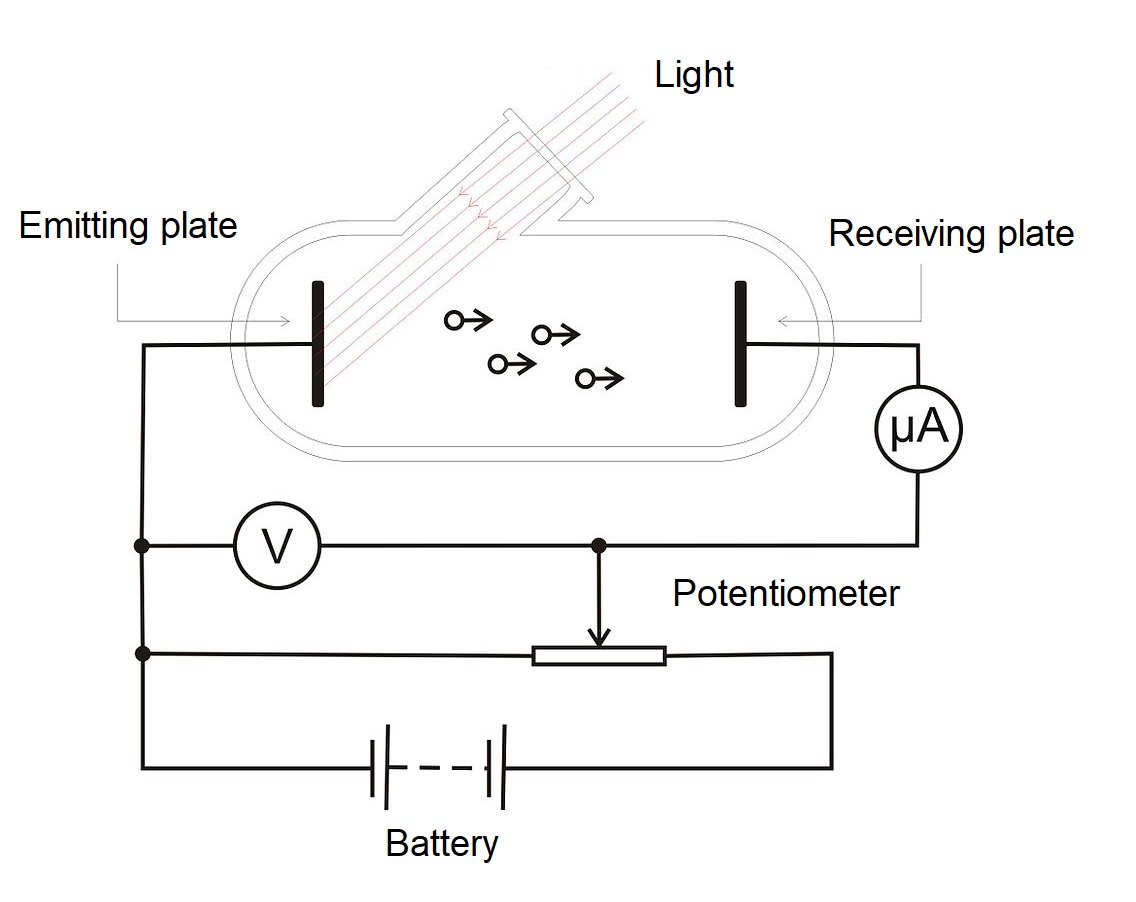
As mentioned above, it consists of a glass vacuum tube with 2 metal electrodes on each end. The electrode is just the metal part connected to the positive or negative pole of a battery outside.
One of the electrode is made of a metal to be tested. When light is shone on it, the light energy electrons may cause electrons to jump out. The scientists probably tried many different experiments. Finally, three of them turns out to finally help them understand what is really the nature of light.
Experiment 1. Threshold frequency.
A battery of a few volts is connected to the electrodes of a glass vaccum tube.
Light of different frequencies are shone on the negative electrode. They found that for lower frequencies, no current flows through the wires outside the tube. This means no electrons jump across the vacuum tube.
They try again at higher frequencies and found that at a certain frequency of light, current starts flowing. They call this the "threshold frequency".
Experiment 2. Stopping potential
In the Experiment 1 above, the scientists found that current can flow even when no voltage is applied to the electrodes. Of course, you cannot just take away the battery - you have to connect the wires between the electrodes.
In this experiment, the battery is reversed and adjusted to a very low voltage. A frequency of light above the threshold frequency is selected.
As the reversed voltage is increased, the current would drop. The reversed voltage where current just drops to zero - is called the "stopping potential".
Einstein's Explanation
In 1905, Albert Einstein came up with an explanation to the above observations. He suggested that:
- light is really made up of tiny packets of energy,
- each packet of light energy is proportional to its frequency,
- an electron on the electrode can only absorb one packet of energy at a time, and
- if the energy is big enough, the electron can jump out of the metal and fly to the positive electrode.
"Big enough" means more than the energy needed to remove an electron from the surface of a metal. Ths energy depends of the particular metal, and is called the work function.
And so, according to nobelprize.org, "The Nobel Prize in Physics 1921 was awarded to Albert Einstein "for his services to Theoretical Physics, and especially for his discovery of the law of the photoelectric effect."
But we students have a few more details to learn. For example, why is the stopping potential is independent of intensity?
One might think that if the light is brighter, then the emitted electrons would have more energy. So we have a use a bigger reverse voltage to stop them.
And here is where the idea of "photon" - a packet of light - comes in. Einstein idea is the light is made up of tiny packets of energy, which we call photons today. and that the energy of a photon is proportional to the frequency of the light.
So the formula for photon energy E is given by a constant h times frequency f. So E = hf. h is called the Planck's constant. It is a very small number : h = 6.63 × 10-34 J s. The unit J s stands for Joule second.
The answer is : brighter light means more photons, so more electrons can be emitted.. Each electron only absorbs one photon's energy. So it still have the same kinetic energy, and can be stopped by the same stopping potential.
More electrons emitted means more current. So current is proportional to intensity of the light.
With the above understanding, we can write down an energy equation.
hf = Φ + ½ m vmax2
Ok, so light, which Thomas Young confirmed in 1801 was a wave, was confirmed about 100 years later by Einstein to be also a particle.
In 1924, Louis de Broglie joined the party. He suggested that maybe electrons, which were definitely particles, might also be a wave.
Now that Einstein has shaken up everyone's understanding of reality, maybe scientist were even more open to new ideas. They went about designing and carrying out experiments to check the idea. And guess what? Surprise, surprise. They managed to produce interference fringes from electron beams.
Interference fringe pattern is the hallmark of waves, as Thomas Young demonstrated. So therefore, electrons are waves also.
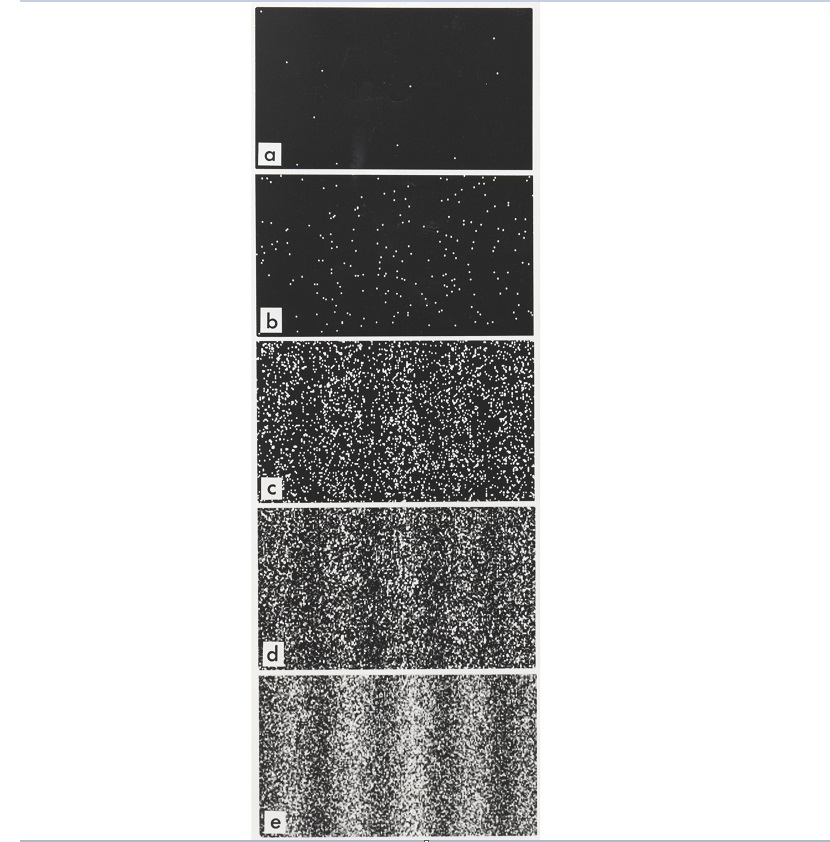
Does this mean that at a dark fringe, electrons cancel each other out and just vanish?
No. The correct understanding - after decades of arguments or discussions among the famous physicists like Einstein etc. - is that when we are not looking, the electrons behave totally like light wave - and is nothing like particles at all. But when they hit the screen and reacted with the photon film chemicals, it is forced to behave as a particle and causes the film to give a tiny white spot.
So the way it behaves depend on whether you are looking ! Ever heard of Schrodinger's cat? A cat is put in a box with a glass of poison. If the cat knock and break the glass, it would die. The box is closed and sound proof, so we cannot hear what is happening inside. So after 1 hour, is the cat dead or alive?
If we look, then the cat is either already dead, or very much alive. But if we don't look? Then it would be dead or alive. So there are two possibilities that exist at the same time. I don't really think this is the right way to understand the wave nature of particles, but the story has become famous in the of history physics.
But before you take this too seriously, Schrodinger probably just made this up as a teaching tool.
de Broglie wavelength
Now that we know electron is also a wave, an obvious question is - what is the wavelength?
de Broglie offered the answer : wavelength λ = h / p
As always, the professional scientists had to carry out experiments to check of this is really the case. It is really hard to imagine how electrons can produce interference fringes. Are some of the electrons going to interfere destructively and just vanish?
As it turns out, the electrons appear to somehow just avoid regions on the screen where they are expected to destructively interfere, and pile up in those regions where they are supposed to interfere constructively - as shown in the image above.
Energy Levels
In a separate development, scientists found that when atoms in a gas gets hot, they emit light with specific frequencies. This is quite different from light form a fire or from the sun, which has a continuous spectrum of a range colours.
Atoms can also emit light when an electric current passes through the gas in a glass vacuum tube.
In both cases, the spectrum looks quite special. When the emitted light through a narrow slit, and then through a prism to separate the different wavelengths - the resulting spectrum looks like a number of lines of specific colours, on a dark background.

What this "line" spectrum shows is that that electrons in atoms cannot have any energy. They have specific energies, like -0.1 J, -0.2 J, -0.3 J, ... but not in between - the so-called energy levels. The result is that when atoms get heated, electrons can gain energy. When they fall back to lower levels, the difference in energy is given out as a particle of light - a photon.
The specific, discrete energy levels also mean that the photons have specific wavelengths. So if the emitted light is sent through a narrow slit to a prism, the image would show a number of lines of different colours. Each line is just an image of the narrow slit, and has a particular wavelength.
We call this collection of lines -- a line spectrum.
These observations further confirm that the wave nature of particles are real.
The above example is called an emission spectrum, since the spectrum comes from light emitted by the atoms.
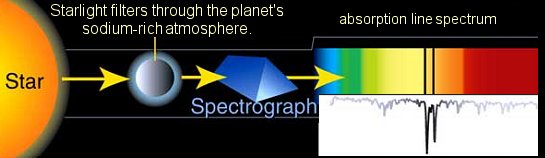
There is another version of the spectrum. When white light passes through a hot gas, some of the frequencies (or colours) can be "absorbed" by the gas atoms. This happens to photons that have just the right amount of energy to cause a electron in the atom to to jump up to a higher energy level.
It would soon fall back down to the original level - by remitting a photon of the same amount of energy. But this would be in some random direction, not ncessarily the original direction of the light.
As a result, some energy would be removed from the original path of the light. When the color goes through a prism and reach the screen, that colour would be less bright, and so looks like a darker line.
This can happen to different energy levels, so dark lines would show up. This spectrum is called an absorption line spectrum. This is and example of missing lines when sunlight passes through sodium vapour.
Recall the formula above for energy of a photon, hf. So the colour of light emitted from or absorbed by a hot vapour depends on the initial energy level E2 and final energy level E1 of an electron in the atom that emits the photon.
So the frequency f is related to the energy levels by
X-ray spectrum
The examples above are on energy levels that can give visible light when electron jump between levels.
For heavier atoms, there are energy levels that have much bigger differences in energies. When electrons jmup between levels, the frequencies absorbed or emitted can be much higher, in the range of X ray spectrum. This is an example.
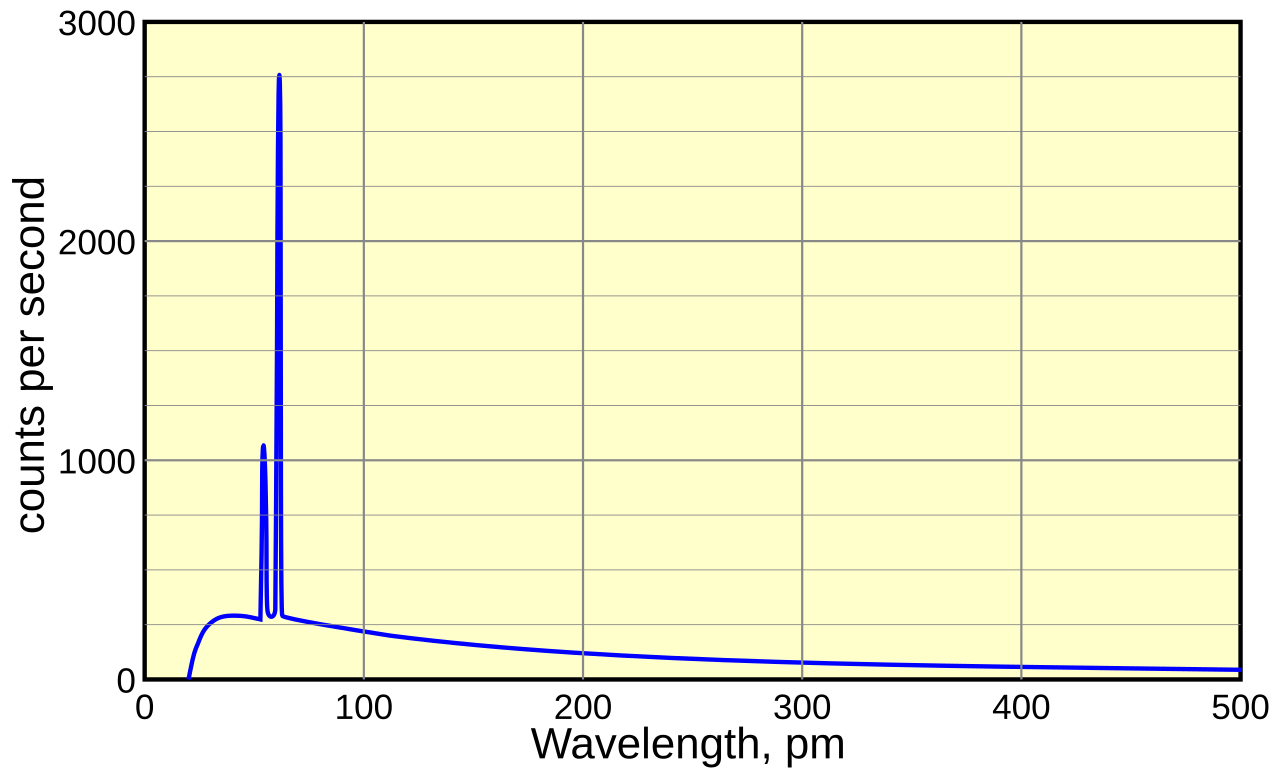
It is the spectrum of the X-rays emitted by an X-ray tube with a rhodium target, operated at 60 kV. The smooth, continuous curve is due to bremsstrahlung, and the spikes are characteristic K lines for rhodium atoms.
Heisenberg uncertainty principle
Finally, we come to a very strange and curious physics principle.
When we do physics experiments, we would naturally want to measure things as accurately as we can. But in physics, there is actually a principle which says that there is a limit to how accurate we can measure distance amd momentum of a particle. And this has nothing to do with how accurate the ruler is. Instead, it is basically like a law of nature.
This principle is called the Heisenberg uncertainty principle :
Let me start with some background examples to explain what this is about. Suppose I have a marble rolling, and I want to measure its position and momentum very accurately.
We can imagine using a ruler, a timer, a video camera, weighing scale, etc. To make it even more accurate, we may use laser devices. Suppose that we have a perfect instrument that can measure the position with zero error, and another perfect instrument that can measure the momentum zero error.
What the Heisenberg uncertainty principle says is :
h = 6.62607015 × 10-34 m2 kg / s, apparently a tiny quantity.
This means that it is not possible to measure them with zero errors at all. Otherwise the left side would be zero, and zero cannot be more than h.
If you look at the value of h above, you can see that we are talking about uncertainties that are very very small by human scale. So no issue measuring our own position and momentum accurately at the same time!
You can learn these concepts and more at Dr Hock's maths and physics tuition.